Celltrion's Antibody Drug Manufacturing: Optimized Facilities and Cutting-Edge Technology
2025.05.14
Antibody biopharmaceuticals are complex biologics derived from recombinant monoclonal antibodies. Since they are produced using living cells, their development requires advanced technology, precise environmental controls, and rigorous quality management.
Celltrion pioneered the world’s first antibody biosimilar, Remsima, and has since developed 12 biosimilars and 2 novel drugs. By managing the entire end-to-end production process in-house—from drug substances to finished products—Celltrion ensures consistent quality, cost efficiency, and supply stability.
The manufacturing process begins with cell line cultivation, where genetically engineered cells are thawed and expanded in seed bioreactors before being transferred to production bioreactors. Leveraging years of expertise, Celltrion employs optimized scaling-up techniques and cultivation methods to prevent cell loss and maximize antibody yield. Tailored cultivation conditions further enhance productivity and protein expression quality.
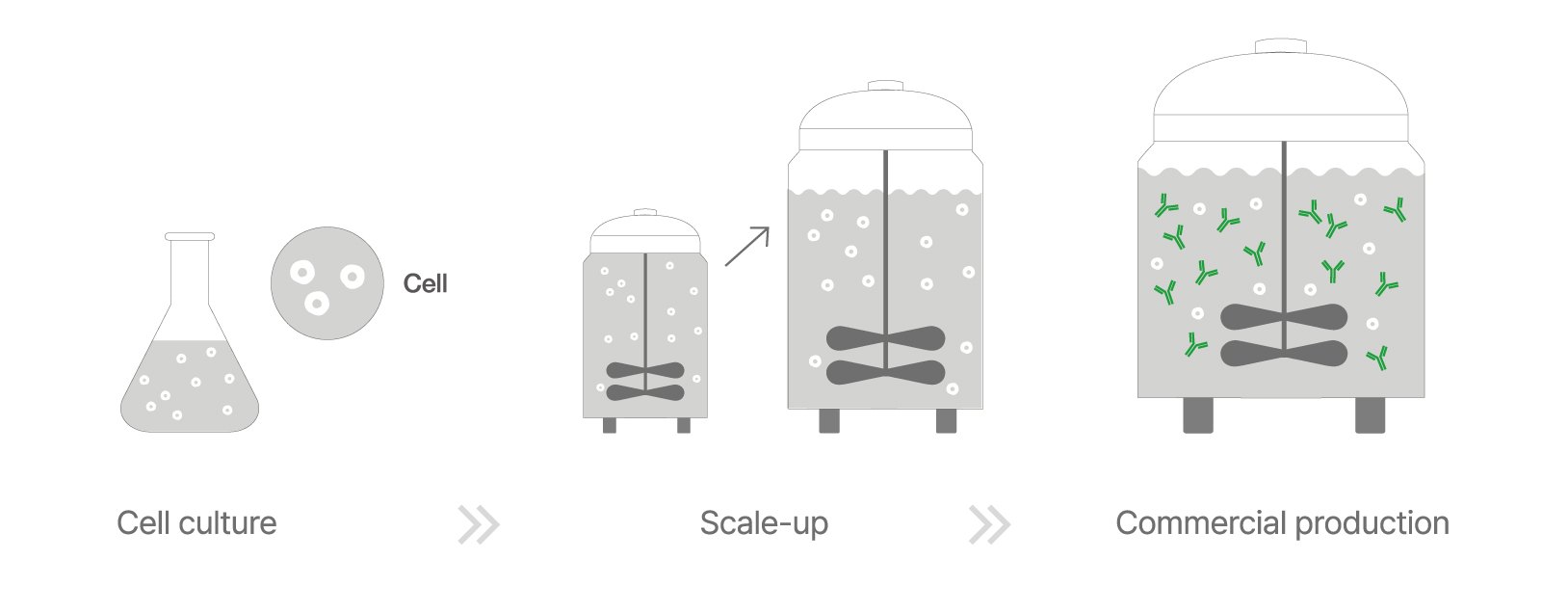
▲ Cell Culture Process
Once cultivation is completed, the culture medium undergoes a three-stage purification process to isolate the desired antibodies. This includes:
Chromatography purification to extract antibodies.
Ultrafiltration (UF) and diafiltration (DF) to remove impurities.
Final filtration to ensure sterility and eliminate contaminants.
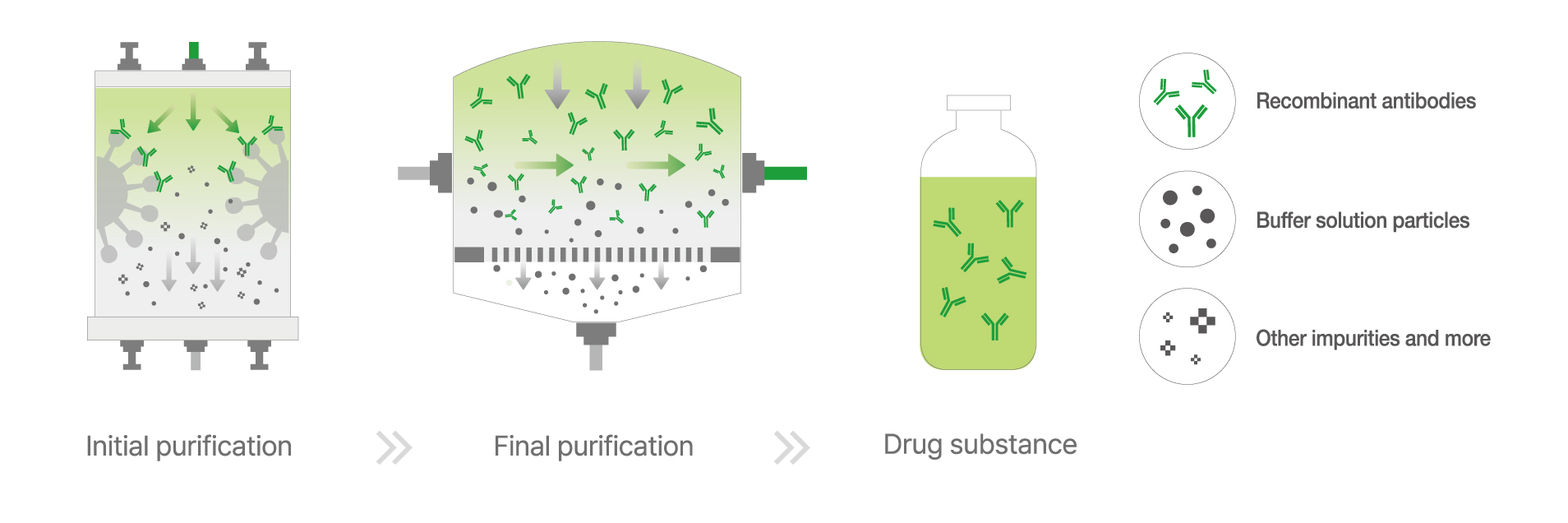
▲ Purification Process
Celltrion holds patents for advanced purification technologies and continuously refines its processes to improve efficiency and quality.
The final stage, known as the fill and finish process, involves transforming drug substances into ready-to-use finished products. This is conducted in ISO-classified cleanrooms, with critical filling steps performed in an ISO 5 environment to maintain sterility. Depending on the formulation, the drug is either kept in liquid form or freeze-dried before being sealed in vials, inspected, labeled, and packaged for global distribution.
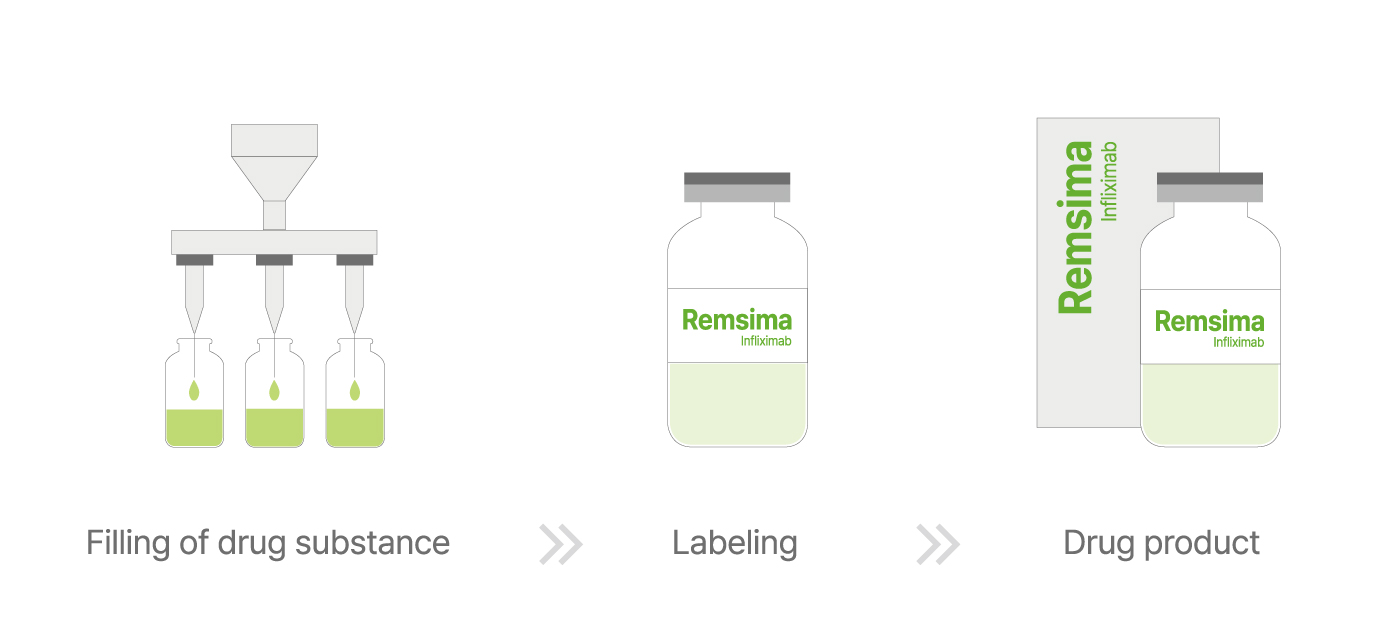
▲ Fill and Finish Process
Biopharmaceutical manufacturing directly impacts drug safety, efficacy, and affordability. By maintaining full in-house production capabilities, Celltrion ensures cost efficiency, quality control, and supply chain stability. As a leading global biopharmaceutical manufacturer, Celltrion remains committed to advancing innovative production technologies to deliver affordable, high-quality treatments to patients worldwide.